NBC
Belviq is the name of a popular weight-loss drug manufactured by Eisai Inc. Lorcaserin is the active ingredient in Belviq. It affects the serotonin receptor in the brain’s hypothalamus region and can help users feel full without overeating. Eisai Inc. voluntarily withdrew Belviq from the United States markets in February of 2020 after the Food and Drug Administration asked them to do so.
Health Risks Associated with Belviq
Health officials first expressed concern for Belviq users in 2012 when it became clear that higher doses of the weigh-loss aid caused hallucinations in some users. These hallucinogenic properties, along with the dependency some users developed for Belviq, led to its classification as a Schedule IV drug by the Drug Enforcement Administration.
There were some concerns early in the life of Belviq that the weight-loss medication could cause cardiovascular problems for some users. These concerns resulted from data collected from users during phase 2 clinical trials. These fears were put to rest by a follow-up study that showed trial participants taking Belviq experienced cardiovascular disease rates similar to study participants given placebos. These results were made available to the public in 2018.
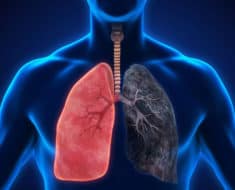
The concern that led to the recent recall became evident after the five-year mark for trial participants. Study participants that took Belviq experienced a higher rate of cancer diagnoses than participants taking a placebo. Lung, colorectal, and pancreatic cancers are among the cancers observed in study participants that took Belviq for at least one year.
What the Recall Means for Users
It is too early to know the numbers of people who developed cancer resulting from Belviq use. But there is still reason to believe these numbers may be low. Medical professionals are also still not sure how Belviq increases the likelihood of cancer diagnosis. However, people who still possess Belviq should stop taking the weight-loss aid and speak with a doctor about what they should do next. There are no special cancer screenings recommended by the FDA at this time.
Alternatives to Belviq
Diet, exercise, and other lifestyle modifications are always the first suggested methods for people with obesity or weight-related medical issues. Belviq is one of several medications available to people who have proven unable to lose weight or sustain weight loss through lifestyle modifications alone.
There are several FDA-approved alternative weight-loss medicines available to consumers. However, it is difficult to say if a customer who experienced success with Belviq will enjoy similar results with another medication. This last fact is relevant because medicines work in their unique way once inside the body. For example, Belviq’s ability to affect serotonin in the brain makes users feel full faster when they enjoy a meal.
Legal Action
Pending Belviq lawsuits are in their early stages. There is no news to report regarding settlements at this time. Consumers diagnosed with lung, colorectal, and pancreatic cancers can file a Belviq lawsuit if they can demonstrate six consecutive months of Belviq use before the diagnosis. It is important to note that the cancers must have originated in the pancreas, lung, or colon. Cancers that spread to these regions from other parts of the body do not qualify for Belviq lawsuits.
The six months of use do not have to happen during the six months immediately preceding the cancer diagnosis. But the use of Belviq should have taken place within seven years before the diagnosis.
Family members of deceased Belviq users can also file a lawsuit against the drug’s manufacturer if their loved one died from one of the above-mentioned cancers after taking Belviq.